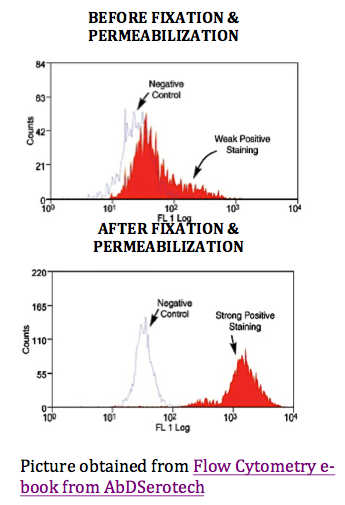
Intracellular Antigen Staining
Effective fixation and permeabilization are the most important parts in intracellular antigen staining as shown as the right peak on the bottom picture:
There are different methods for fixation and permeabilization used. The most important is to access the internal epitope of interest without disturbing the morphology of the cell.
Different methods of fixation and permeabilization available at Abcam website:
- Formaldehyde followed by detergent
Detergents:
- Triton or NP-40 (can partially dissolve the nuclear membrane)
- Tween 20, Saponin and Leucoperm (mild membrane solubilizers)
- Formaldehyde followed by methanol
- Methanol followed by detergent
- Acetone fixation and permeabilization

Pre-staining procedure:
- Add 100 µl of fixative. Incubate for 10 minutes at required temperature.
- Add 100µl detergent based permeabilizing agent and incubate in the dark at room at room temperature for 15 minutes.
- Wash the cells by adding 2ml of PBS (containing 0.1% triton or other permeabilizing detergent), centrifuge at 300g (2000 rpm) for 5 minutes, discard supernatant and re-suspend the pellet in the volume remaining.
- Follow antibody-staining procedure.

Links
CMtO Intracellular Staining Guide
Intracellular Antigen Staining
All work performed by the Roy J. Carver Biotechnology Center (CBC) should be acknowledged in scholarly publications, posters, and presentations. Proper recognition allows us to measure the impact of our work and supports our initiatives in obtaining sponsored funding. In addition, any CBC personnel who make a substantial intellectual or experimental contribution are deserving of further recognition as co-author.