Sample Submission Requirements:
Our facility offers a full range of services for library construction and sequencing with the NovaSeq X Plus, MiSeq, PacBio Revio and the 10x Genomics single-cell.
We have extensive experience in denovo genome and transcriptome sequencing, genome and transcriptome resequencing, profiling of gene expression, comparative genomics and other applications using RNA-Seq, DNA_Seq, DNA methylome, ATAC-Seq, Cut&Run, single-cell and other applications. Turnaround time is typically one to three weeks from the day we receive the samples or libraries.
Sequencing Options
| Single-reads or paired-reads: |
Library fragments can be sequenced from one end (single-reads) or from both ends (paired-ends). Single-reads are typically used for genome resequencing, gene expression profiling and small RNA sequencing. Paired-reads are most commonly used for de novo assembly, characterization of splice variants, and other applications that benefit from more information from the sequenced fragments. |
| Typical Read Lengths and Yields: |
NovaSeq X Plus:
NovaSeq 6000:
MiSeq i100 and MiSeq:
PacBio REVIO:
|
Library construction:
Libraries in our facility are constructed with kits from Illumina using UNIQUE DUAL INDEXES (UDI's) to avoid index switching. Our RNAseq libraries are strand-specific.
All constructed libraries are quantitated with fluorometry (Qubit), checked for proper size distribution and complete primer/adaptor removal on a Fragment Analyzer, diluted to 5nM, and quantitated by qPCR prior to sequencing.
Sample submission requirements:
|
Sample Type |
Minimum Quantity needed for library construction |
Volume |
Special instructions |
Options |
|
Genomic DNA for shotgun libraries |
1ng to 1μg |
20-50μl in 10mM Tris |
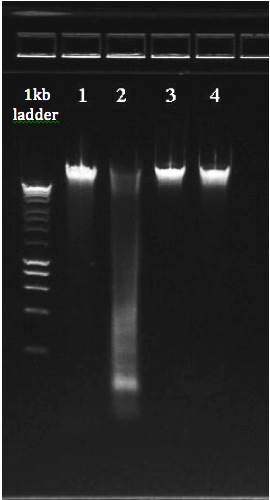
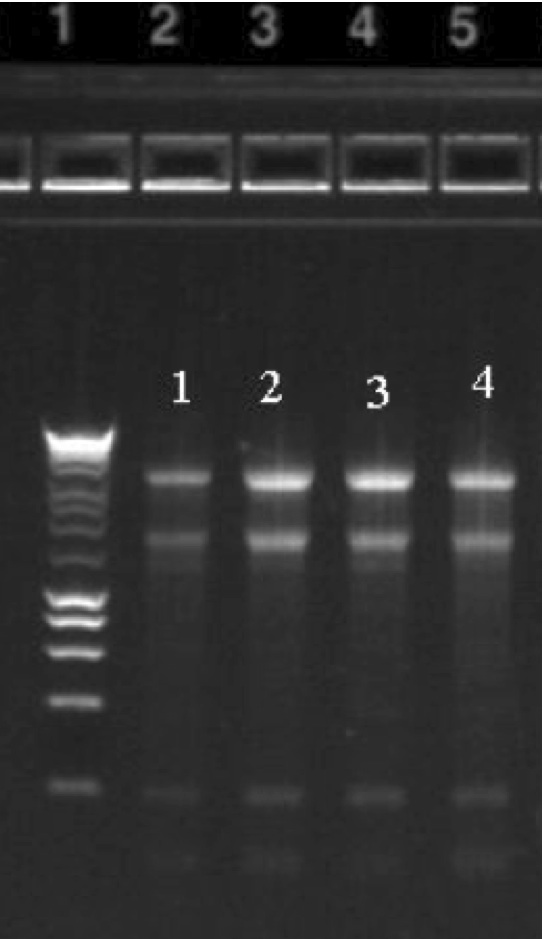
* Quantitation with fluorometry (Qubit or Picogreen) is critical. * DNA can also be quantitated on a 1% agarose gel next to a 1 Kb mass DNA ladder. * Submit a picture with an aliquot of the DNA on a 1% gel next to a ladder before sending the sample. See below for examples of high quality DNA. * DNA should be clean of contaminants, salts, etc |
Fragment sizes can be customized to a tight size or wide range, i.e. libraries can be constructed with average fragment sizes of 180bp, 500bp, 400-800bp, etc. |
|
Total RNA |
1ng to 1μg |
20 to 50μl of RNAse-free water |
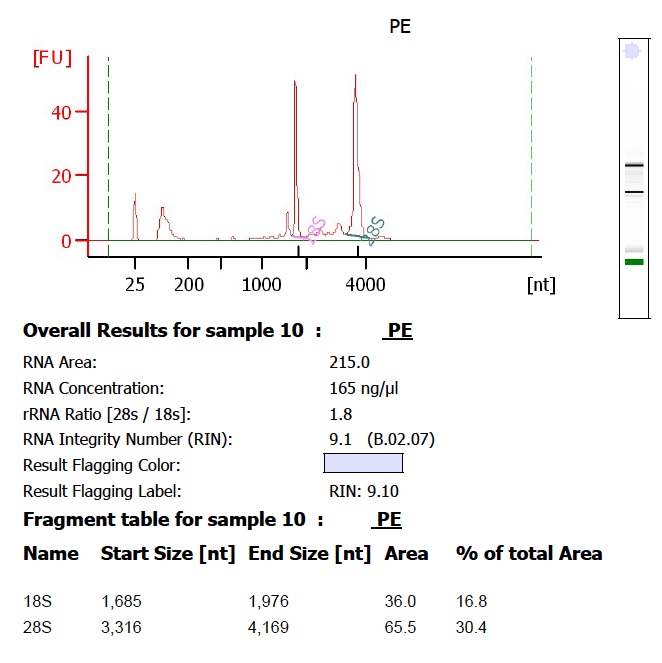
*RNA must be treated with DNase. *Run RNA either on a 1% agarose gel next to a DNA ladder or on a bioanalyzer RNA chip and submit picture or .pdf file. See below for examples of high quality total RNA. |
Customer submits high-quality total RNA, free of contaminating DNA. |
|
ATAC or ChIP DNA |
1ng+ |
10μl in 10mM Tris buffer |
*DNA must be sonicated to a size of 100-600bp (<600bp) before ChIP procedure. * Quantitation with fluorometry (Qubit or Picogreen) is critical. * Send gel image or bioanalyzer trace of sonicated input DNA if available. |
|
|
Small RNA |
100ng of total RNA |
20 to 50μl, in RNAse-free water |
*See instructions for Total RNA above. *It is critical that total RNA is not purified with columns that remove fragments <100nt. *Kits that enrich for the small RNA fraction can also be used. |
|
|
Single-Cell RNA-Seq (10x Genomics) See this page for full details.
|
500k -1M cells | at least 20μl |
*Elimination of dead cells is critical, viability should be >70%. Cells can be processed through a Dead Cell removal kit if required. *Concentration should be at least 5000 cells/ul (5M/ml). *Starting cell suspension will be counted (live/dead measure included), washed twice in PBS with 0.04% BSA, and counted again to ensure proper cell capture. |
Each library will be made from 500 to 10,000 cells, depending upon your project need. Required sequencing is a minimum of 50,000 2x100nt paired-reads per cell; 100k reads/cell and 2x150nt length reads are recommended. |
| Genomic DNA for PacBio REVIO | 5ng to 2ug |
~ 50 to 100ul in 10mM Tris buffer |
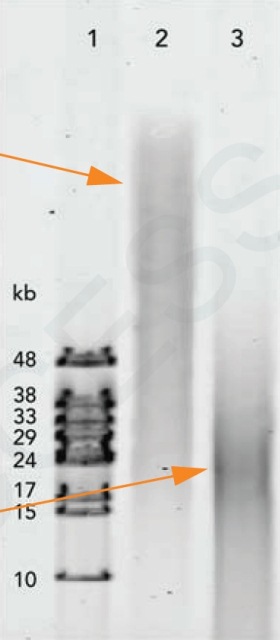
DNA should be in fragments > 50kb. DNA must be free of proteins, polysaccharides and polyphenols and any other contaminants. The A260/280 and A260/A230 ratios should be 1.8 or higher |
HiFi (error corrected) reads: 10-30kb |
| Genomic DNA for Oxford-Nanopore |
1-2μg 500ng for rapid libraries |
20-100μl in 10mM Tris buffer | DNA must be free of proteins, polysaccharides and polyphenols and any other contaminants. The A260/280 and A260/A230 ratios should be 1.8 or higher | libraries can be made with an average fragment length of 8-15kb (higher output) or with longer fragments. |
| RNA for direct RNA sequencing on Oxford-Nanopore | at least 500ng of polyA+ RNA | 2--100μl of water | If 500ng of polyA+ RNA are not available, mRNA can be converted to cDNA and PCR-amplified |
Customers submitting their own libraries:
Libraries must either be compatible with the read1, index and read 2 primers used by the current NovaSeq/MiSeq chemistry or be submitted with at least 20ul of 100uM custom Read1, index and Read2 primers. Libraries should be submitted pooled and at 10nM dilutions if possible.
For multiplexed libraries, please submit a final pool at 10nM (i.e. do not submit individual libraries before pooling). Dilutions to 10nM should be based on quantitation by fluorometry and average size determined with a bioanalyzer/fragment analyzer. Enter these values in this attached calculation sheet
Once submitted, we will quantitate your pooled libraries with qPCR prior to sequencing.
Gel and Bioanalyzer images of genomic DNA and Total RNA:
Please contact Dr. Alvaro Hernandez, Director of DNA Services (aghernan@illinois.edu) at 217-244-3480 or Chris Wright, Associate Director of DNA Services (clwright@illinois.edu) at 217-333-4372 to discuss ways the staff can be of assistance in achieving your project goals or to receive a quote for your project, submission forms, grant support or other information needed.
All work performed by the Roy J. Carver Biotechnology Center (CBC) should be acknowledged in scholarly publications, posters, and presentations. Proper recognition allows us to measure the impact of our work and supports our initiatives in obtaining sponsored funding. In addition, any CBC personnel who make a substantial intellectual or experimental contribution are deserving of further recognition as co-author.